Synuclein research 1: pathophysiological effects on mitochondrial integrity
The synuclein protein family consist of three closely related proteins, a-, b-, and g-Synuclein. They all show very little secondary or tertiary structure in solution, and are thus members of the ever-growing family of “unfolded” proteins. a-Synuclein can form protein aggregates under certain conditions, similar to other proteins linked to “aggregopathies” like Tau, Aß or Huntingtin. b-Synuclein was long thought to be an anti-aggregating counterpart to a-Synuclein, but we have recently demonstrated that it forms proteinase K-resistant aggregates in the rodent brain very similar to a-Synuclein (Taschenberger at al, Ann Neurol, 2013). a-Synuclein is intimately linked to the etiology of Parkinson´s disease (PD), as mutants and gene multiplications of a-Synuclein are directly causative for parkinsonian symptoms, and aggregations of a-Synuclein are found in all idiopathic PD patients. In order to better understand how a- but also ß-Synuclein inflict on neuronal physiology we use optical live imaging and biochemical tools in cultured primary neurons and in the rodent brain. An example of how we think pathophysiology is induced in mitochondria is illustrated below (deduced from Toloe at al, Front Mol Neurosci, 2018).
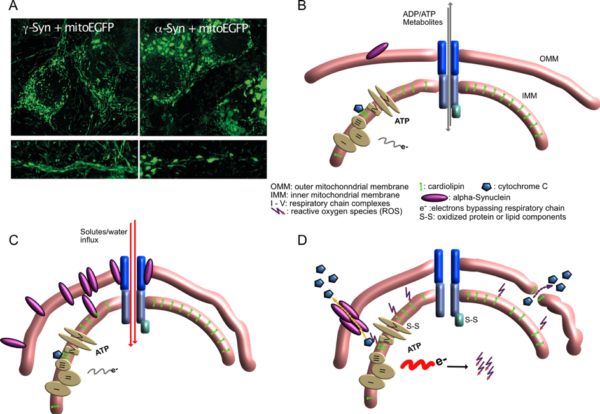
Mitochondrial lesions caused by a-synuclein
- A) Neurons overexpressing g-Synuclein show thin and elongated mitochondria in cytoplasm as well as in neurites (left panel), while mitochondria of a-Synuclein overexpressing neurons are swollen and condensed.
- B) Situation at the interface of inner and outer mitochondrial membrane under control conditions, when little a-Synuclein interacts with mitochondrial membranes.
- C) Under conditions of a-Synuclein overabundance it binds to mitochondrial membranes, causing enhanced membrane curvature and/or increased solute influx. Although mitochondria appear morphologically distorted at this stage, they are perfectly fine with respect to ATP production, ion handling capacities or ROS production.
- D) After longer-term interaction of a-Synuclein with mitochondrial membranes, ROS production increases, probably causing release of cytochrome C from its cardiolipin anchor (this part is speculative so far), resulting in even more loss of electrons from the respiratory chain and further oxidative damage. Finally, cytochrome C becomes released from mitochondria, either through a-Synuclein-generated pores or through damaged OMM, leading to induction of apoptosis-like neuronal cell death.